What is the cell potential for an electrochemical cell at 298 K with a copper metal electrode immersed in a 0.30 M copper sulfate solution in one compartment and a copper metal electrode immersed in a 1.5 M copper sulfate solution in the other compartment? Cu2+(aq) + 2 e- Cu(s) , 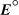 = +0.34 V
= +0.34 V
A) "+0.34 V"
B) "-0.34 V"
C) "+0.021 V"
D) "+0.36 V"
E) "More information is needed to decide."
Correct Answer:
Verified
Q92: The Energizer Bunny in the television commercial
Q94: The standard cell potential for the
Q95: Does pH have an effect on
Q96: Calculate Ecell for an electrochemical cell
Q97: Calculate Ecell for an electrochemical cell
Q99: When a voltaic cell reaches equilibrium,_
A)
Q100: What is true when a battery (voltaic
Q101: In a classroom demonstration of a redox
Q103: The electrodes on batteries are labeled +
Q118: If 15 g of aluminum from an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents