The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology.The overall reaction,which is given below,has a G° value of -237 kJ/mol.What is the standard cell potential for this fuel cell? H2(g) + 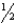 O2(g) H2O(
O2(g) H2O(  )
)
A) 2.46 V
B) 4.91 V
C) 1.23 V
D) 2.460 V
E) 1.50 V
Correct Answer:
Verified
Q92: Electrolytic processes are used in a variety
Q136: How long would it take to electroplate
Q137: A hydrogen fuel cell depends on _
A)fusion
Q138: Which of A-D is a disadvantage in
Q139: Methanol fuel cells depend on the
Q141: A 1.5 V alkaline battery is constructed
Q142: Write the cell diagram for the
Q143: For the electrochemical cell based on the
Q144: How many coulombs of charge are
Q145: What is the cell potential for a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents