Multiple Choice
Identify the expression that results after multiplying Ka of HA and Kb of A-.
A) 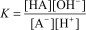
B) 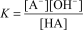
C) 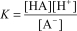
D) 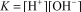
E) 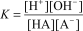
Correct Answer:
Verified
Related Questions
Q57: What is the pH of a 0.500
Q58: The concentration of acetic acid (pKa =
Q60: In an experiment, 0.438 L of 0.152
Q63: The pH of a 0.250 M
Q65: A cup of coffee has a
Q66: The acid ionization equilibrium constant,Ka,describes which
Q69: A solution with a pOH of
Q71: What is the pH of a
Q72: A 0.100 M monoprotic weak acid solution
Q73: The base ionization constant Kb describes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents