The molar solubility,s,for Al(OH) 3 is written as __________.
A) 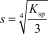
B) 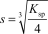
C) 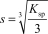
D) 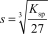
E) 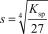
Correct Answer:
Verified
Q45: Stalactites-the long, icicle-like formations that hang from
Q166: The solubility product for Al(OH)3 is written
Q167: The molar solubility,s,for Ba3(PO4)2 is written as
Q168: Glycolic acid,which is a monoprotic acid and
Q170: Vitamin C is a monoprotic weak acid,which
Q172: The solubility product for Ca3(PO4)2 is written
Q173: Suppose a 1.0 L solution containing
Q174: Suppose a 1.0 L solution containing
Q175: The solubility product for CaF2 is written
Q176: A 200.0 mL solution of 0.40
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents