Arsenic acid is a triprotic acid in water,ionizing in the following sequential steps:
H3AsO4 + H2O H2AsO4- + H3O+
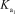 = 5.5 *10 - 3
= 5.5 *10 - 3
H2AsO4- + H2O HAsO42- + H3O+
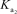 = 1.7 *10-7
= 1.7 *10-7
HAsO42- + H2O AsO43- + H3O+
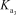 = 5.1 * 10-12
= 5.1 * 10-12
What is the approximate pH of a 0.80 M Na3AsO4 solution?
Correct Answer:
Verified
Q215: What is the pH of a
Q216: What is the pH of 8.6
Q217: A 0.025 L sample of oven cleaner
Q218: What is the pH of a
Q219: Arsenic acid is a triprotic acid
Q221: Calculate the pH of a solution
Q222: Ksp for silver sulfate is 1.2
Q223: You wish to use precipitation to
Q224: Suppose 10.0 mL of 2.00 M
Q225: Ksp for magnesium fluoride is 6.5
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents