For the equilibrium,2 PH3(g) P2(g) + 3 H2(g) ,the equilibrium partial pressures are 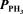 = 0.022 atm,
= 0.022 atm,  = 0.289 atm,and
= 0.289 atm,and 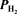 = 0.867 atm at 873 K.Calculate Kp.
= 0.867 atm at 873 K.Calculate Kp.
A) 0.0585
B) 17.1
C) 0.0441
D) 2.50 10-3
E) 389
Correct Answer:
Verified
Q13: Which of the following is true for
Q21: Consider the following equilibrium: CH4(g)+ H2O(g)
Q22: Consider the following equilibrium: CH4(g)+ 2
Q24: For the chemical equilibrium aA +
Q26: The equilibrium constant for the formation
Q27: Write the equilibrium expression for the
Q28: For the chemical equilibrium aA +
Q29: Consider the equilibrium,2 NOCl(g)
Q30: Which of the following statements regarding the
Q38: An increase in the number of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents