For the equilibrium 2 PH3(g) P2(g) + 3 H2(g) ,Kc = 0.0776 at 873 K.What is Kp at 873 K for the equilibrium 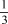 P2(g) + H2(g)
P2(g) + H2(g) 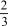 PH3(g) ?
PH3(g) ?
A) 7.36
B) 0.136
C) 2.34
D) 0.182
E) 40.4
Correct Answer:
Verified
Q39: For the chemical equilibrium aA +
Q40: Consider the following equilibrium: ClF3(g)
Q41: Two students measure the equilibrium constant for
Q42: There are many reactions involving nitrogen,oxygen,and
Q43: A series of four equilibrium steps
Q45: Chromic acid is a diprotic acid:
Q46: Which of the following statements regarding the
Q47: Write the expression for the reaction
Q48: For the equilibrium 2 HI(g)
Q60: If the reaction quotient Q has a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents