For the equilibrium N2(g) + O2(g) 2 NO(g) ,Kp = 0.0017 at 2300 K.At a given point,the partial pressures of the gases are 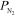 =
= 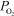 = 0.660 atm and
= 0.660 atm and 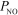 = 0.0272 atm.Which of the following statements is true?
= 0.0272 atm.Which of the following statements is true?
A) Q < K so the reaction will continue to make more products.
B) Q > K so the reaction will consume products to make more reactants.
C) Q = K so the system is at equilibrium.
D) The value of K will decrease until it is equal to Q.
E) The value of K will increase until it is equal to Q.
Correct Answer:
Verified
Q41: In equilibrium expressions, the concentrations of pure
Q45: What happens to the equilibrium between NO2(g)
Q60: The following reactions at 1000.0 K
Q64: For the equilibrium 2 NOBr(g)
Q65: If the temperature of an endothermic reaction
Q67: Which of A through D is NOT
Q69: For the equilibrium H2(g)+ S(s)
Q70: Consider the equilibrium CH4(g)+ H2O(g)+ 206
Q72: Which of the following is true regarding
Q74: Addition of reactants to a chemical reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents