The 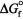 of atomic oxygen is 230.1 kJ/mol.Determine the equilibrium constant for the following reaction at standard thermodynamic conditions: O2(g) 2 O(g) .
of atomic oxygen is 230.1 kJ/mol.Determine the equilibrium constant for the following reaction at standard thermodynamic conditions: O2(g) 2 O(g) .
A) 4.7 10-41
B) 2.3 10-61
C) 2.1 10-81
D) 2.1 1040
E) 4.5 1080
Correct Answer:
Verified
Q64: If K for a reaction is
Q113: As the temperature of an endothermic
Q114: For the phase transition H2O(
Q116: The
Q117: A sketch of the free energy versus
Q119: A sketch of the free energy for
Q120: A sketch of the free energy for
Q121: As T approaches infinity,ln K approaches _
A)infinity.
B)zero.
C)
Q122: Given the following two reactions and
Q123: Write an expression for the equilibrium constant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents