Given the following two measurements of the equilibrium constant for a reaction,calculate H for the reaction. 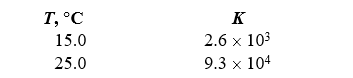
A) 256 kJ/mol
B) -271 kJ/mol
C) 3250 kJ/mol
D) -3250 kJ/mol
E) +271 kJ/mol
Correct Answer:
Verified
Q84: Chemical equilibrium arises from the _ of
Q96: A qualitative interpretation of the effect
Q124: A sealed tube containing an equilibrium
Q125: A reaction is run under conditions
Q126: When plotting ln K vs.1/T,a linear
Q127: For the equilibrium 2 OF2(g)
Q130: Consider the equilibrium CO(g)+ 2 H2(g)
Q131: Gaseous carbon monoxide and hydrogen gas
Q132: The values of Q134: For the equilibrium N2(g)+ 3 H2(g)![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents