Using the thermodynamic data below,determine the equilibrium constant for the conversion of oxygen to ozone,3 O2(g) 2 O3(g) ,at 2.00 103 K.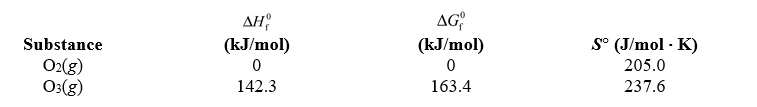
A) 2.04 107
B) 5.44 10-14
C) 2.67 10-8
D) 2.91 10-9
E) 3.65 10-12
Correct Answer:
Verified
Q97: Write an expression for the equilibrium constant
Q115: If Q for a reaction is greater
Q131: The prediction of linearity in a
Q135: A plot of ln K vs.1/T
Q136: Consider the equilibrium,2 NOBr(g)
Q137: For the reaction of hydrogen and
Q144: Consider the reaction of nitrogen and
Q145: Equilibrium constants for two reactions are
Q153: Consider a reaction at a point in
Q184: Values for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents