Estimate Kp at 500 K to three significant figures for the reaction N2(g)+ 3 H2(g) 2 NH3(g),given the following data:
Kp at 298 K = 6.1 105  (NH3,g)= -16.5 kJ/mol
(NH3,g)= -16.5 kJ/mol 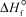 (NH3,g)= -46.1 kJ/mol
(NH3,g)= -46.1 kJ/mol
Correct Answer:
Verified
Q93: For a reaction to be spontaneous, the
Q108: How and why are the expressions for
Q111: One mole of solid ammonium carbamate (NH4CO2NH2)
Q114: Students often write expressions for the equilibrium
Q144: Consider the reaction of nitrogen and
Q145: Equilibrium constants for two reactions are
Q149: For the equilibrium,CO(g)+ 2 H2(g)
Q150: Nitrogen monoxide molecules can react to
Q151: Hydrofluoric acid,HF,dissociates in water according to
Q154: Calculate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents