For the reaction CH4(g) + 4 NO(g) 2 N2(g) + CO2(g) + 2 H2O(g) ,the rate of the reaction in terms of the change in nitrogen monoxide concentration would be written as __________
A) 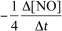
B) 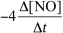
C) 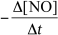
D) 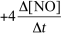
E) 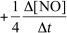
Correct Answer:
Verified
Q1: The order in which ozone, nitrogen monoxide,
Q4: Which of the following statements regarding catalytic
Q5: Indicate which of the following compounds is
Q6: NO2 contributes to the "brown haze" associated
Q7: For the reaction 2 A +
Q13: At what time of day are ozone
Q14: Which of the following is NOT
Q24: HI dissociates to form I2 and
Q34: If the rate of formation of
Q37: In the combustion of methane, CH4(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents