For the reaction CH4(g) + 4 NO(g) 2 N2(g) + CO2(g) + 2 H2O(g) ,the rate of the reaction in terms of the change in nitrogen concentration would be written as __________
A) 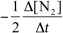
B) 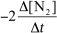
C) 
D) 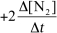
E) 
Correct Answer:
Verified
Q1: The order in which ozone, nitrogen monoxide,
Q2: What is the device in an automobile
Q9: Large cities often issue ozone advisories. At
Q10: Which of the following is NOT important
Q14: Which of the following is NOT
Q16: Smog created by the interaction of sunlight
Q19: For the reaction 2 A +
Q24: HI dissociates to form I2 and
Q34: If the rate of formation of
Q37: In the combustion of methane, CH4(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents