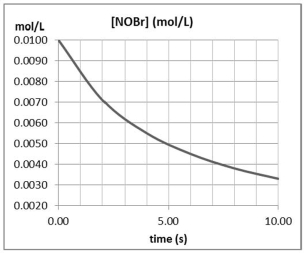
Approximately how many times faster or slower is the average reaction rate during the first four seconds (t = 0 to t = 4) than the second four seconds (t = 4 to t = 8) for the following reaction? 2 NOBr(g) Br2(g) + 2 NO(g) 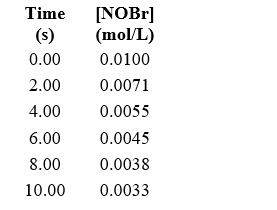
A) about 1.4 times faster
B) about 1.4 times slower
C) about 2.64 times slower
D) about 2.6 times faster
E) The average rate is constant.
Correct Answer:
Verified
Q25: If the rate of formation of
Q26: The rate of disappearance of HI
Q27: Which of the following statements regarding the
Q28: If the average rate of reaction
Q30: For the reaction 4 NH3(g)+ 7
Q31: For the reaction 3 I-(aq)+ H3AsO4(aq)+
Q32: Which of the following does the initial
Q33: Which of the following statements is
Q33: If the average rate of reaction
Q34: The rate of disappearance of HI
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents