The following figure shows Arrhenius plots for four different reactions.Which reaction has the greatest temperature dependence? 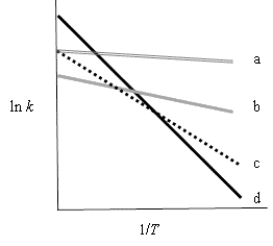
A) double solid (a)
B) gray (b)
C) dotted (c)
D) solid (d)
E) more information is needed
Correct Answer:
Verified
Q63: The linear form of the Arrhenius equation
Q63: The linear form of the Arrhenius equation
Q90: The linear form of the Arrhenius
Q98: The rate constant for the reaction
Q99: The rate constant for the decomposition
Q100: The half-life for the second-order decomposition
Q101: The energy profiles for four different reactions
Q102: The following figure shows Arrhenius plots for
Q106: Which of the following statements regarding collision
Q107: The following energy profiles for four different
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents