The slope and intercept of an Arrhenius plot made for the first-order decomposition reaction, H2O2 H2O + 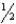 O2,are -12,500 K and 33.02,respectively.What is the value of k at 303 K?
O2,are -12,500 K and 33.02,respectively.What is the value of k at 303 K?
A) 1.47 103 s-1
B) 8.15 1014 s-1
C) 2.19 1014 s-1
D) 7.00 10-3 s-1
E) 2.65 10-4 s-1
Correct Answer:
Verified
Q106: Which of the following describes a reaction
Q110: In an elementary step of a reaction
Q113: A reaction mechanism consists of a series
Q114: A proposed mechanism for the photodecomposition
Q118: The reaction NO2(g) + CO(g)
Q125: Determine the activation energy,Ea,of the decomposition
Q127: Given the following data for the
Q128: Given the following data for the
Q131: Given the following data for the
Q132: The mechanism for the reaction 2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents