Given the Following Data for the Reaction A B,determine the Activation Energy,Ea,of the Reaction
Given the following data for the reaction A B,determine the activation energy,Ea,of the reaction. 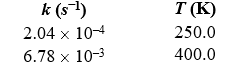
A) 2.34 kJ/mol
B) 19.4 kJ/mol
C) 38.2 kJ/mol
D) 2.02 kJ/mol
E) 18.6 kJ/mol
Correct Answer:
Verified
Q95: Which of the following must be true
Q110: In an elementary step of a reaction
Q113: A reaction mechanism consists of a series
Q118: The following figure shows Arrhenius plots for
Q119: The energy profiles for four different reactions
Q120: Which of the following statements regarding transition
Q122: Given the following data for the
Q125: Determine the activation energy,Ea,of the decomposition
Q127: Given the following data for the
Q128: Given the following data for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents