The hydrolysis of methyl acetate in water,CH3COOC2H5(aq)+ H2O( 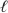 ) CH3COOH(aq)+ C2H5OH(aq),is pseudo-first order in dilute aqueous solutions with a rate constant of 2.00 10-3 min-1.What is the value of the second-order rate constant,where the concentration of water is no longer assumed to be 55.5 M?
) CH3COOH(aq)+ C2H5OH(aq),is pseudo-first order in dilute aqueous solutions with a rate constant of 2.00 10-3 min-1.What is the value of the second-order rate constant,where the concentration of water is no longer assumed to be 55.5 M?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q147: The linear form of the Arrhenius equation
Q149: The linear form of the _ is
Q152: Briefly explain the features of a pseudo-first-order
Q160: In an elementary step of a reaction
Q161: In a catalyzed reaction, the activation
Q192: For the second-order reaction,NO(g)+ F2(g)
Q193: The oxidation of iodide by peroxodisulfate,S2O82-(aq)+
Q194: The isomerization reaction of cyclopropane to
Q197: Given the following data,estimate the rate
Q199: Suppose the surface-catalyzed hydrogenation reaction of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents