Chlorine atoms react with methane,CH4(g)+ Cl(g) HCl(g)+ CH3(g).The rate constant for the reaction is measured at 298,303,308,and 313 K.
(a)Using the plot of ln k vs.1/T,calculate the activation energy and the frequency factor of the reaction.
(b)Estimate the rate constant at 318 K using the Arrhenius equation. 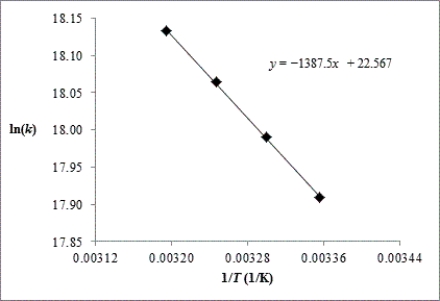
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q105: The linear form of the Arrhenius equation
Q112: The linear form of the Arrhenius equation
Q125: The device in automobiles that has decreased
Q143: In a(n) _ step of a reaction
Q145: Briefly explain how the instantaneous rate of
Q181: The reaction of hydroxyl radical with
Q183: Suppose the surface-catalyzed hydrogenation reaction of an
Q186: The reaction NO2(g)+ CO(g)
Q187: The isomerization reaction of cyclopropane to
Q188: The isomerization reaction of cyclopropane to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents