Multiple Choice
Which of the following probably has the highest entropy at 298 K?
A) 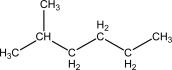
B) 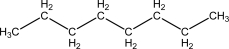
C) 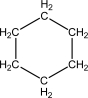
D) 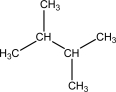
E) 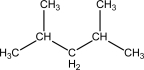
Correct Answer:
Verified
Related Questions
Q9: The entropy change in a system
Q13: Which of the following statements is a
Q17: The following figures represent distributions of two
Q18: Given one mole of each of the
Q23: Which of the following are listed in
Q27: Which of the following statements regarding absolute
Q27: The molar entropies of carbon monoxide
Q29: Which of the following is in the
Q31: At 0 K, the entropy of a
Q45: Indicate which of the following has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents