In a biochemical reaction,A + B C with 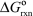 = 30 kJ/mol.Which of the following reactions might be effectively coupled to this reaction so that it becomes more spontaneous?
= 30 kJ/mol.Which of the following reactions might be effectively coupled to this reaction so that it becomes more spontaneous?
(I) C + D B + E 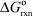 = -40 kJ/mol
= -40 kJ/mol
(II) C + D B + E 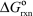 = +40 kJ/mol
= +40 kJ/mol
A) I only
B) II only
C) I or II
D) Neither I nor II can increase spontaneity.
E) No coupling is required,as the reaction is already spontaneous.
Correct Answer:
Verified
Q81: The enthalpy and entropy of fusion
Q115: What is the overall standard free
Q117: Consider the reaction HgO(s)+ Zn(s)
Q118: Consider the freezing of water at
Q119: Which of the following statements regarding glycolysis,glucose
Q121: Carbon atoms can be found in a
Q122: Hydrogen iodide can theoretically be made
Q158: Give an example of a spontaneous and
Q182: The standard entropy of N2(g) is 191.5
Q186: What is a microstate and how are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents