Determine the standard free energy change for the reaction H2(g)+ 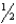 O2(g) H2O(l)from the data given.
O2(g) H2O(l)from the data given.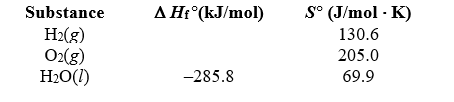
Correct Answer:
Verified
Q141: Estimate the free energy change for
Q142: Hydrogen iodide can theoretically be made
Q143: The standard molar enthalpy of fusion
Q144: What is the overall standard free-energy
Q146: The entropy of fusion for ice
Q148: Determine the normal melting point of benzoic
Q149: The change in standard molar entropy,
Q150: Suppose a fermentation process occurs in
Q165: What are the signs of
Q167: Draw a graph of entropy versus temperature
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents