Create the Born-Haber cycle and calculate the lattice energy of sodium fluoride from the following data: Ionization energy of Na: 496 kJ/mol
Electron affinity of F: -328 kJ/mol
Energy to vaporize Na: 108 kJ/mol
F2 bond energy: 160 kJ/mol
Energy change for the reaction:
Na(s) + 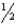 F2(g) NaF(s) ; H = -575 kJ
F2(g) NaF(s) ; H = -575 kJ
A) 931 kJ/mol
B) -931 kJ/mol
C) -1011 kJ/mol
D) 1011 kJ/mol
E) -851 kJ/mol
Correct Answer:
Verified
Q1: Use the following data to calculate
Q2: Which of the following will require the
Q4: Which of the following processes is
Q5: Create the Born-Haber cycle and calculate the
Q7: Use the following data to calculate
Q8: Which one of the ionic compounds below
Q10: Which of the following statements regarding the
Q21: Which of the following requires the smallest
Q29: Which one of the ionic compounds below
Q140: Which of the following is NOT typically
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents