The following graph shows the speed distributions for four different gases,all at the same temperature.Which of the curves is for the lightest gas? 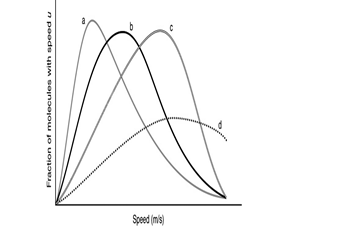
A) a
B) b
C) c
D) d
E) more information is needed
Correct Answer:
Verified
Q28: At a given temperature,the effusion rate of
Q29: About how long would it take
Q30: At a given temperature,nitrogen gas effuses 1.194
Q32: Which of the following figures is the
Q34: Air,which is primarily nitrogen and oxygen,does
Q35: Calculate the average kinetic energy of
Q36: At a given temperature,the rate at which
Q37: Which set of gases is listed
Q38: Air is primarily nitrogen and oxygen,but noble
Q118: Which of these gases (Ar, N2O,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents