The following diagrams illustrate the flow of energy (q) and work (w) in different processes.In which ones does the internal energy of the system DEFINITELY increase? Assume the magnitudes of q and w are equal.
A) 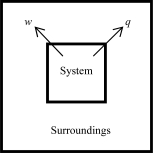
B) 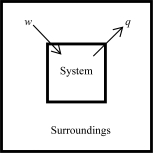
C) 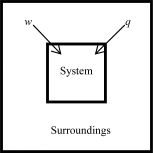
D) 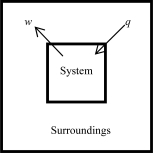
E) none of these
Correct Answer:
Verified
Q2: Energy that an object has by virtue
Q9: What is the change in internal
Q10: What is the change in the
Q11: Which of following will always increase the
Q11: Which of the following statements about energy,
Q15: Work requires
A)a use of potential energy.
B)a release
Q15: According to the first law of thermodynamics,which
Q16: Thermochemistry is the study of how _
Q17: Which of the following bar charts shows
Q22: Internal energy is defined as _
A)the total
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents