Given that molar enthalpy of combustion of acetylene (C2H2,26.04 g/mol) is -1299 kJ/mol at 25°C and 1 atm,which of the following is false? 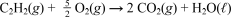
A) The enthalpy of the reactants is lower than that of the products.
B) The amount of heat released is proportional to the amounts of reactants.
C) When 104 g C2H2 combusts,about 5200 kJ of heat is released.
D) The enthalpy of combustion per gram acetylene is-49.9 kJ/g.
E) To form 2 mol C2H2 and 5 mol O2 from 4 mol CO2 and 2 mol H2O,2598 kJ of energy is required.
Correct Answer:
Verified
Q79: How much energy is needed to
Q80: In an experiment,10.0 g of ice
Q81: When butane (C4H10,58.14 g/mol)is burned in
Q82: In an experiment,0.250 kg water at 95.0°C
Q83: In an experiment,30.0 g of metal is
Q85: A 550 g solution at 27.27°C is
Q86: A 225 g piece of iron [CP
Q87: You hold a 50 g sphere of
Q88: The dissolution of cesium hydroxide in
Q89: Suppose the brain needs to metabolize
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents