Given the standard enthalpies of formation for the following substances,determine the change in enthalpy for the combustion of 1.0 mol of butane. Substance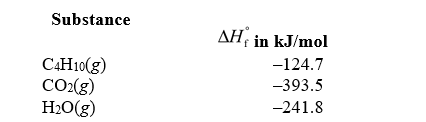
A) +2658 kJ/mol
B) -5317 kJ/mol
C) -2658 kJ/mol
D) +5317 kJ/mol
E) -3647 kJ/mol
Correct Answer:
Verified
Q90: Determine the change in enthalpy for
Q117: Which of the following hydrocarbons has the
Q118: Fuel density is _
A)the cost of energy
Q125: Use the bond energies in the
Q127: For which of the following is the
Q128: Lightweight camping stoves typically use a mixture
Q129: Which of the following substances,a-d,will release the
Q131: Which of the following does NOT
Q134: Determine the change in enthalpy for
Q135: Lightweight camping stoves typically use a mixture
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents