Determine the standard enthalpy of formation for NO given the following information about the formation of NO2 under standard conditions,and 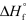 (NO2) = + 33.2 kJ/mol.
(NO2) = + 33.2 kJ/mol.
2 NO(g) + O2(g) 2 NO2(g) Hrxn = -114.2 kJ
A) 90.3 kJ/mol
B) -90.3 kJ/mol
C) -147.4 kJ/mol
D) -180.6 kJ/mol
E) -73.7 kJ/mol
Correct Answer:
Verified
Q85: Which statement regarding combustion of a sample
Q107: Fuel value is _
A)the cost of energy
Q117: Which of the following hydrocarbons has the
Q120: A food sample was burned in a
Q121: The complete combustion of carbon compounds
Q122: Coal is converted into cleaner,more transportable fuels
Q125: Use the bond energies in the
Q127: For which of the following is the
Q128: Lightweight camping stoves typically use a mixture
Q129: Which of the following substances,a-d,will release the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents