The Enthalpy of Combustion of Hydrogen Gas Is -285 104 Kg Satellite with Enough Kinetic Energy to Reach the Provide
The enthalpy of combustion of hydrogen gas is -285.8 kJ/mol.To provide a 2.20 104 kg satellite with enough kinetic energy to reach the escape velocity of Earth (roughly 11.2 km/s) ,how many kilograms of H2 must be burned? (Kinetic energy = 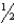 mv2,where mass is in kg and velocity is in m/s; 1 J = kg . m2/s2)
mv2,where mass is in kg and velocity is in m/s; 1 J = kg . m2/s2)
A) 1.95 104 kg
B) 4.83 103 kg
C) 2.49 105 kg
D) 9.75 103 kg
E) 8.62 102 kg
Correct Answer:
Verified
Q87: Ethanol (CH3CH2OH) has been suggested as
Q105: The enthalpy of combustion of table sugar
Q114: Ethanol (CH3CH2OH) has been suggested as
Q147: A 7.50 g marshmallow is placed in
Q148: A Snickers bar contains 12 g fat,33
Q154: Which of the following fuels has
Q155: Which of the following fuels has
Q160: Suppose you eat a one-third-pound hamburger without
Q166: Give a brief definition of thermochemistry and
Q175: Give a brief definition of internal energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents