When ammonia gas is dissolved in water,which of the following best represents the resulting solution?
A) NH3(aq) + H2O(  ) NH4+(aq) + OH-(g)
) NH4+(aq) + OH-(g)
B) NH3(aq) + H2O(  ) N-(aq) + H3O+(aq)
) N-(aq) + H3O+(aq)
C) NH3(aq) + H2O(  ) HNO3(aq)
) HNO3(aq)
D) NH3(aq) + H2O(  ) NH2O(aq) + H2(g)
) NH2O(aq) + H2(g)
E) NH3(aq) + H2O(  ) H3O+(aq) +
) H3O+(aq) + 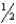 N2(g)
N2(g)
Correct Answer:
Verified
Q45: What is the molar concentration of sodium
Q46: Which of the following is a strong
Q47: When sulfur trioxide gas is dissolved
Q48: Which of the following is true regarding
Q49: In its reaction with water,phenol (C6H5OH)acts as
Q51: Identify the acid in the following
Q52: Which of the following statements about a
Q53: If 50.0 mL of a 0.10 M
Q54: Identify the acid in the following
Q55: Ethylamine,C2H5NH2,acts as a weak base in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents