In an aqueous solution under basic conditions,ozone (O3) reacts with iodide (I-) and water to form iodine (I2) ,hydroxide (OH-) ,and oxygen (O2) .What is the sum of the stoichiometric coefficients in the balanced reaction equation? O3(g) + I-(aq) + H2O 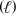 I2(aq) + OH-(aq) + O2(g)
I2(aq) + OH-(aq) + O2(g)
A) 6
B) 8
C) 10
D) 12
E) 14
Correct Answer:
Verified
Q23: In a demonstration of the complete combustion
Q26: Sulfur dioxide from coal-fired power plants combines
Q32: The acid-base reaction between phosphoric acid, H3PO4,
Q36: What are the stoichiometric coefficients for oxygen
Q37: Hydrazine (N2H4)can decompose to form nitrogen gas
Q41: The space shuttle uses liquid hydrogen
Q43: The most abundant metal in Earth's crust
Q44: As ammonium hydrogen carbonate (NH4HCO3,79.1 g/mol)is heated,it
Q54: One form of elemental sulfur is a
Q57: The combustion of ethanol (CH3CH2OH, 46.1 g/mol)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents