Potassium Perchlorate (KClO3)oxidizes Sucrose (C12H22O11)according to the Following Unbalanced Reaction
Potassium perchlorate (KClO3) oxidizes sucrose (C12H22O11) according to the following unbalanced reaction.What are the coefficients in the balanced chemical equation in the order in which it is written? KClO3(s) + C12H22O11(s) KCl(s) + CO2(g) + H2O 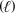
A) 4,1,4,12,11
B) 4,1,4,12,22
C) 2,1,2,12,11
D) 8,1,8,12,11
E) 8,2,8,12,11
Correct Answer:
Verified
Q4: Air bags in cars inflate when an
Q26: Sulfuric acid reacts with a vanadium
Q26: Sulfur dioxide from coal-fired power plants combines
Q27: Extracting copper from sulfide ores can
Q29: Which statement,A-D,regarding photosynthesis is NOT correct? In
Q30: Chlorine atoms in the atmosphere can
Q32: The acid-base reaction between phosphoric acid, H3PO4,
Q36: What are the stoichiometric coefficients for oxygen
Q39: Diesel fuel for automobiles and trucks is
Q58: Glucose (C6H12O6) is oxidized by molecular oxygen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents