Mathematically combine the following three reactions to arrive at the following overall balanced equation for the complete combustion of the tricyclic antidepressant,imipramine (C19H24N2).Show your work and write your final equation with whole-number coefficients.Assume nitrogen is completely oxidized to NO2.
(i)C(s)+ O2(g) CO2(g)
(ii)H2(g)+ 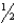 O2(g) H2O
O2(g) H2O  (iii)N2(g)+ 2 O2(g) 2 NO2(g)
(iii)N2(g)+ 2 O2(g) 2 NO2(g)
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q106: What is the difference in the chemistry
Q112: Calcium carbide (CaC2, 64.10 g/mol) reacts with
Q114: Write the balanced reaction equation for the
Q115: Calcium hydride (CaH2,42.10 g/mol)is so reactive
Q118: Nitrogen and oxygen can react to form
Q119: Ammonia (NH3, 17.04 g/mol) is industrially produced
Q120: Nitrogen and oxygen can react to form
Q123: For which of the following compounds are
Q124: Bullvalene is 92.26% C and 7.74% H;
Q139: Traumatic acid, an organic acid with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents