Which of the substances a-e in the following figure has the weakest intermolecular forces? 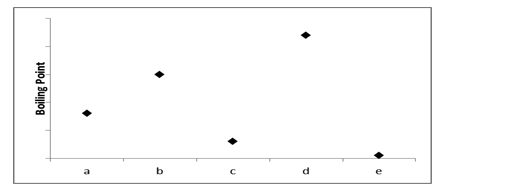
A) a
B) b
C) c
D) d
E) e
Correct Answer:
Verified
Q3: Which of the following compounds will have
Q14: A hydration sphere forms around an ion
Q15: Which of the following would you predict
Q16: Identify the compound that is most likely
Q17: The relative energies (strengths)of the intermolecular forces
Q18: Which of the following compounds is capable
Q21: Polarizability refers to _
A)the ease with which
Q24: Indicate which of the following molecules exhibits
Q26: For a molecule to exhibit dipole-dipole interactions,
Q30: Indicate which of the following compounds will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents