Which of these molecules have a dipole moment? 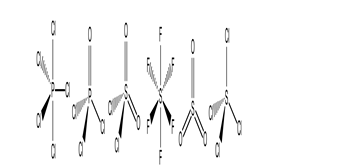
A) PCl5 and SiCl4
B) POCl3,SO2Cl2,and SO3
C) POCl3 and SO2Cl2
D) PCl5,POCl3,SOCl2,SO3,and SiCl4
E) PCl5,SF6,SO3,and SiCl4
Correct Answer:
Verified
Q50: What is the hybridization of arsenic in
Q50: Which of the following compounds has the
Q51: Which of the following contains polar bonds
Q52: What is the hybridization of carbon in
Q52: Benzene (C6H6) is a cyclic, nonpolar molecule.
Q53: Which statement regarding hybrid orbitals and molecular
Q54: Which of the following shows the orientation
Q56: Which of the following statements regarding
Q57: Which statement regarding
Q59: Which of the following compounds has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents