Oxygen has two common molecular anions: peroxide (O22-) and superoxide (O2-) .Use the MO energy level diagram below to identify which one of the following statements is NOT correct. 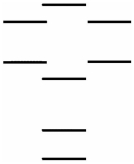
A) The bond order of the peroxide is 1.
B) The superoxide has a shorter bond than the peroxide.
C) Like O2,the peroxide is paramagnetic.
D) The superoxide has a stronger bond than the peroxide.
E) Oxygen,O2,has a stronger bond than either of these oxides.
Correct Answer:
Verified
Q106: Which of the following molecules is paramagnetic?
A)F2
B)O2
C)C2
D)Be2
E)C2
Q107: Which of the following molecules or ions
Q108: Which of the following molecules or ions
Q109: Determine the bond order of S2-.
A)3
B)2.5
C)2
D)1.5
E)1
Q110: Which type of molecular orbital contains
Q113: Which one of the following statements
Q114: Use MO theory to predict the bond
Q115: Which of the following statements regarding molecular
Q117: Which type of molecular orbital has
Q126: Which one of the following molecules has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents