Determine the bond order of the PO molecule based on oxygen's 2p electrons and phosphorus's 3p electrons.Use the MO diagram shown below. 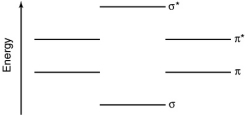
A) 2
B) 2.5
C) 1.5
D) 1
E) 0.5
Correct Answer:
Verified
Q113: Predict the bond order of the NO+
Q122: Determine the bond order of the NO+
Q125: Carbonyl dihalides (COX2 with X = I,Cl,or
Q126: Predict the following three bond angles and
Q128: Use MO theory to predict the bond
Q129: Which of the following statements regarding bonding
Q130: Use energy levels of diatomic molecules derived
Q131: Consider the series: NO2+,NO2,and NO2-.Draw one Lewis
Q132: Boron nitride is being investigated in frontier
Q178: Draw a structure showing the geometry of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents