Identify the hybridization of atomic orbitals for atoms N1,N2,C1,C2,and C3 in caffeine,which is shown below.Explain why you think this molecule is planar or nonplanar. 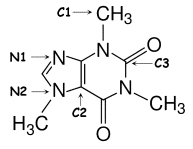
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q135: Draw the Lewis structure of BrF5.Give the
Q140: ICl3 and SO3 have one central atom.Do
Q143: The amide functional group is the fundamental
Q146: A Lewis structure of aspirin without the
Q147: Identify the local molecular geometry and hybridization
Q149: Answer the following questions about the
Q150: Consider the structure of glyceraldehyde (nonbonding
Q151: Identify the hybridization of atomic orbitals for
Q152: A Lewis structure of aspirin without the
Q153: Identify the hybridization of the atomic orbitals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents