Which of the following statements is false regarding the Lewis structure of trichloroacetic acid,CH2Cl3O2? A partial bonding framework is given. 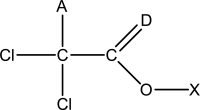
A) The atom at position "X" is hydrogen.
B) In the correct and complete Lewis structure,the atom at position "D" violates the octet rule.
C) Another double bond is required in order for all of the atoms to attain full valence shells.
D) The oxygen atom shown has two pairs of nonbonding electrons.
E) In the correct and complete Lewis structure,all of the chlorine atoms have nonbonding pairs of electrons.
Correct Answer:
Verified
Q33: Indicate which of the following molecules has
Q59: Indicate which molecule contains the largest number
Q67: How many nonbonding electrons are there in
Q85: Which of the following bonds could definitely
Q86: How many covalent bonds are there in
Q88: Identify the molecule or ion that contains
Q89: How many nonbonding electron pairs are there
Q92: Vinegar is a solution of acetic acid
Q93: Which of the following is most likely
Q95: Which of the following bonds could be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents