What type of bonds are the external N-O bonds (see arrows) in dinitrogen pentoxide (N2O5) ? An incomplete bonding framework is provided. 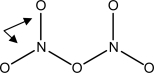
A) both single
B) one single and one double
C) both between a single and double
D) both double
E) between a single and a triple
Correct Answer:
Verified
Q23: In general, resonance _ electrons and _
Q93: The formal charge of an atom in
Q99: How many lone-pair electrons are on each
Q114: In which of the following molecules does
Q126: The formal charge on the nitrogen atom
Q129: What is the formal charge of the
Q131: Resonance structures indicate that _
A)there is more
Q134: What is the term used for odd-electron
Q134: Which structure for dinitrogen sulfide (N -
Q135: What is the formal charge on the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents