Use the formal charge as a criterion to identify the Lewis structure below that is the best in representing the bonding in sulfur dioxide.
A) 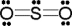
B) 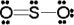
C) 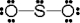
D) 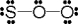
E) 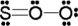
Correct Answer:
Verified
Q74: Bond length and bond strength are _
A)
Q83: The formal charge on the sulfur atom
Q88: Which one of the following molecules or
Q111: Which of the following is the longest
Q112: How many valence electrons surround the xenon
Q114: What is the bond order of the
Q145: Which of the following compounds is likely
Q147: Which of the following bonds will be
Q150: The formal charge on the phosphorus atom
Q153: Which of the following has the strongest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents