Estimate the enthalpy of reaction for 2 H2(g) + O2(g) 2 H2O(g) using bond energies.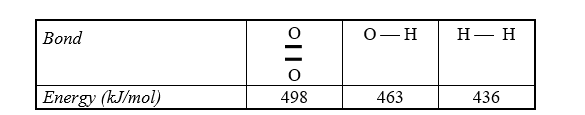
A) "482 kJ/mol"
B) "444 kJ/mol"
C) "-444 kJ/mol"
D) "-482 kJ/mol"
E) "-918 kJ/mol"
Correct Answer:
Verified
Q127: The evaporation of seawater gives a mixture
Q127: Rank the following ionic compounds in order
Q131: In a chemical reaction, bonds are broken
Q132: What is the correct name for CuCl2?
Q140: What is the chemical formula for manganese(IV)
Q159: What are the names for the following
Q160: How many nonbonding electrons are on the
Q161: What is the correct name for Mg3(PO4)2?
Q162: What is the chemical formula for ammonium
Q168: Ethanethiol and dimethyl sulfide share the same
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents