Three reasonable resonance structures are shown for the cyanate ion,OCN-.Assign formal charges to each atom.Which structure,if any,contributes most heavily to the resonance hybrid structure,and why?
(A) 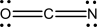 (B)
(B) 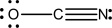 (C)
(C) 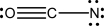
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q90: Which of the following bonds is considered
Q91: Using the positions of the atoms in
Q123: Draw the Lewis structure for the nitrate
Q125: Identify which of the following molecules has
Q136: Do you expect the nitrogen-oxygen bond length
Q142: Draw two reasonable Lewis structures for sulfurous
Q152: Describe the relationship between bond order, bond
Q180: Draw the Lewis structures of PCl3 and
Q187: Two reasonable Lewis structures for sulfurous acid
Q191: Based on electronegativities,which bond of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents