Which of the transitions in the hydrogen atom energy-level diagram shown here is not possible? 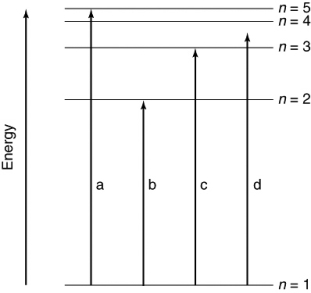
A) all are possible
B) a
C) b
D) c
E) d
Correct Answer:
Verified
Q43: Electromagnetic radiation with a frequency of 8.6
Q45: Which of the following transitions emits the
Q52: What is the shortest wavelength of light
Q53: You think you have identified a
Q54: How much energy would be required
Q58: Determine the wavelength of the line in
Q58: What wavelength of light is required to
Q59: Which transition in a hydrogen atom will
Q60: What is the difference in energy
Q61: The energy of a one-electron atom or
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents