The energy change for an electronic transition in a one-electron atom or ion (H,He+,Li2+,etc.) from ninitial to nfinal is given by 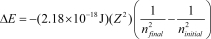 ,where Z is the atomic number. How much energy is required to ionize a ground-state He+ ion (nfinal = ) ?
,where Z is the atomic number. How much energy is required to ionize a ground-state He+ ion (nfinal = ) ?
A) 2.18 10-18 J
B) 4.36 10-18 J
C) 8.72 10-18 J
D) 1.09 10-18 J
E) 5.45 10-19 J
Correct Answer:
Verified
Q49: Which of the following statements regarding the
Q59: Which transition in a hydrogen atom will
Q60: What is the difference in energy
Q61: The energy of a one-electron atom or
Q62: An electron _ will have the
Q63: What is the velocity of an
Q65: What is the de Broglie wavelength
Q67: Calculate the de Broglie wavelength of
Q68: If the electron (9.11
Q69: What is the de Broglie wavelength
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents