Which of the following statements about electron spin and the spin quantum number,ms,is NOT true?
A) The idea of electron spin was introduced to explain pairs of lines in atomic emission spectra.
B) The electron creates a magnetic field because it is a charged particle moving through space.
C) The spin of en electron is quantized.
D) Electrons in the same orbital can have the same spin.
E) The spin quantum number can only have one of two values,+ 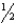 and -
and - 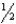 .
.
Correct Answer:
Verified
Q76: How many orbitals are possible for the
Q79: The mathematical description of an electron as
Q81: How many orbitals exist for the quantum
Q84: What are the principal and angular momentum
Q85: A shell consists of all _
A)electrons with
Q89: If the principal quantum number is seven
Q91: Which subshell has only five orbitals?
A)
Q95: Which of the following statements regarding orbitals
Q98: Which of the following states that each
Q100: A certain shell is known to have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents