Which of the following sets of quantum numbers is NOT allowed?
A) n = 1,  = 1,m
= 1,m  = 0,ms = -
= 0,ms = - 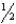
B) n = 3,  = 0,m
= 0,m  = 0,ms = +
= 0,ms = + 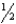
C) n = 3,  = 1,m
= 1,m  = -1,ms = -
= -1,ms = - 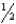
D) n = 5,  = 4,m
= 4,m  = 0,ms = +
= 0,ms = + 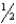
E) n = 6,  = 3,m
= 3,m  = 2,ms = -
= 2,ms = - 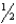
Correct Answer:
Verified
Q101: Which of the following statements about the
Q103: Which of the following is the correct
Q104: What information do boundary-surface representations of orbitals
Q105: Which of the following is a possible
Q106: Which of the following represents an s
Q108: You synthesize an atom that appears to
Q109: Which set of quantum numbers could be
Q110: Which of the following is the correct
Q111: Which of the following orbitals is the
Q112: Which of the following statements about atoms
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents