Which of the following orbitals is the lowest in energy (assuming they are all in the same shell) ?
A) 
B) 
C) 
D) 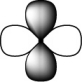
E) 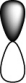
Correct Answer:
Verified
Q93: The size of a typical atomic orbital
Q106: Which of the following represents an s
Q107: Which of the following sets of quantum
Q108: You synthesize an atom that appears to
Q109: Which set of quantum numbers could be
Q110: Which of the following is the correct
Q112: Which of the following statements about atoms
Q113: Which of the following is the correct
Q114: Which set of quantum numbers could be
Q116: Which of the following elements has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents