Which of the following statements regarding degenerate orbitals is correct?
A) Orbitals sharing the same principal and angular momentum quantum numbers are degenerate.
B) Orbitals sharing the same magnetic quantum number are degenerate.
C) Electrons with the same value of n are equal in energy in all atoms.
D) Orbitals sharing the same 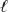 value are degenerate.
value are degenerate.
E) As the effective nuclear charge increases,all orbitals in a shell become equal in energy.
Correct Answer:
Verified
Q82: In quantum mechanics, an atomic orbital _.
A)provides
Q88: Which of the following is NOT an
Q97: What Q99: Which of the following statements about the Q100: Which combination of quantum numbers would be Q101: Which of the following statements about the Q103: Which of the following is the correct Q104: What information do boundary-surface representations of orbitals Q105: Which of the following is a possible Q106: Which of the following represents an s![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents